Training structure
Faculty of Science
Program
OPTION 1
4 creditsChoose 2 out of 3
Supplements in solution chemistry
2 creditsCrystallography I
2 creditsThermodynamics and phase equilibria
2 credits
Basic elements of radioactivity
2 creditsPolymers
2 creditsChemistry of solutions applied to actinides
2 creditsAdvanced inorganic materials
2 creditsSolutions, colloids, interfaces
2 creditsLiquid NMR spectroscopy and X-ray diffraction
2 creditsChemometrics, statistical data analysis, experimental design
2 creditsMethodology for characterizing materials
2 creditsCoordination chemistry and organic chemistry
2 creditsProfessional projects – project monitoring
8 credits
Chemistry at the scale of indicators - Radiochemistry
2 credits2-4 month internship (bibliography included)
10 creditsInnovative synthesis and extraction processes
2 creditsRadiation protection / radiation-matter interaction
2 creditsOPTION 2
4 creditsChoose 2 out of 3
Fundamentals of Process Engineering
2 creditsHybrid and structured materials
2 creditsContainment materials
2 credits
Liquid-liquid extraction: kinetics and thermodynamics
2 creditsHigh-temperature chemistry
2 creditsCommunication and professional integration
2 creditsFuel cycle: from mining to waste management
2 creditsCoordination chemistry of f-elements
2 credits
Supplements in solution chemistry
Level of education
Bachelor's degree
ECTS
2 credits
Training structure
Faculty of Science
This course on solution chemistry aims to introduce the various concepts necessary for studying complex liquid mixtures used in separation chemistry. The approach taken is mainly thermodynamic. In particular, we explain the role of concentration effects, beyond the ideal laws that apply only to dilute solutions.
CM: 12 H
Tutorial: 8 hours
Crystallography I
Level of education
Bachelor's degree
ECTS
2 credits
Training structure
Faculty of Science
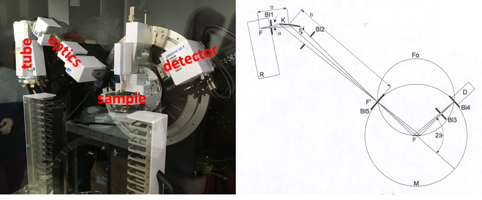
This lecture, delivered entirely in English, provides a basic introduction to crystallography and electron diffraction for beginners. X-ray diffraction is an important characterization technique in modern chemistry; the majority of crystalline structures in inorganic and organic solids have been solved using this method. It is therefore important for all students to understand its basic concepts and instrumentation. The course provides explanations and principles of X-ray diffraction together with the geometry and symmetry of X-ray patterns. In addition to the interaction principles of X-rays and matter, it covers how to obtain quantitative intensities for single crystal and powder diffraction patterns. It naturally includes an understanding of lattice planes and the reciprocal lattice concept together with the Ewald sphere construction. Furthermore, it provides a basic understanding of the Fourier transform relationship between the crystalline structure and the diffracted intensities, as well as the reciprocal lattice concept.
Electron diffraction is a complementary technique to X-rays that provides information in terms of symmetry and geometry on the materials studied. In this course, we will therefore approach the description of the method for obtaining electron diffraction patterns and their interpretation. We will be able to obtain the lattice parameters, the reflection conditions, as well as the groups of possible spaces.
This lecture also serves as the introductory part to the lecture Electron Microscopy and Crystallography II.
CM: 14
TD: 6
Thermodynamics and phase equilibria
Level of education
Bachelor's degree
ECTS
2 credits
Training structure
Faculty of Science
- Review of thermodynamics of single-component systems.
- Basic concepts of thermodynamics in multicomponent systems. Chemical potential, Gibbs-Duhem relation, variance.
- Concepts related to thermal analysis techniques used to construct binary/ternary diagrams: ATG, ATD, and DSC
- Construction and interpretation of binary phase diagrams based on thermodynamic quantities. Gibbs free enthalpy, pressure, and temperature diagrams as a function of the composition of the binary mixture. Liquid-liquid, liquid-vapor, and solid-liquid mixtures.
- Phase transformations: first- and second-order transitions, critical points. Examples.
- The supercritical state: definition, thermodynamic properties, most widespread industrial applications.
- Construction and interpretation of ternary phase diagrams: variance, definitions of ternary eutectic, first and second order peritectic, isothermal section, study of alloy cooling.
Hourly volumes:
CM: 13
TD: 7
Basic elements of radioactivity
Level of education
Bachelor's degree
ECTS
2 credits
Training structure
Faculty of Science
This teaching unit covers the various basic elements needed to understand natural and artificial radioactivity phenomena. It aims to establish all the concepts related to decay phenomena, natural radioactive families and their associated environmental consequences, dating methods, methods of producing radionuclides and their use in various fields, as well as anthropogenic contributions. Various examples from industry, nuclear energy, radiochemistry, geochemistry, and nuclear medicine will be used to illustrate the basic concepts covered.
Hourly volumes:
CM: 12 p.m.
Tutorial: 8 hours
Polymers
Level of education
Bachelor's degree
ECTS
2 credits
Training structure
Faculty of Science
Polymers are all around us: we eat them, we wear them, and we use them to construct extremely complex buildings. From mature technologies to the most innovative materials, polymers are a crucial building block for constructing the world of tomorrow. In this course, we will cover several aspects such as the controlled synthesis of polymers and cross-linked materials, surface modification using polymers, some characterization tools suitable for polymers, and finally a last section developing the latest advances involving polymers.
Hourly volumes:
CM: 1:00 p.m.
Tutorial: 7 hours
Chemistry of solutions applied to actinides
Level of education
Bachelor's degree
ECTS
2 credits
Training structure
Faculty of Science
A general approach to the chemistry of actinide elements in aqueous solution will be developed through concepts of thermodynamics and kinetics, redox potentials, hydrolysis, and complexation. To support these concepts, concrete examples from industry, recycling, and the environment will be discussed.
Hourly volumes:
CM: 11 a.m.
Tutorial: 9 a.m.
Advanced inorganic materials
Level of education
Bachelor's degree
ECTS
2 credits
Training structure
Faculty of Science
The HAC720C module covers "advanced inorganic materials" in five main sections. Thefirst section is devoted to general information on inorganic materials and discusses structure-property relationships, with particular attention paid to chemical bonding, real crystals, and polycrystalline solids. The different classes of inorganic materials are described. Thesecond part focuses on ceramic materials (definitions and properties) and their synthesis (raw materials including clays, shaping, drying and debinding, sintering); a distinction is made between traditional ceramics and technical ceramics (synthesis methods for oxide and non-oxide ceramics). Thethird part covers glass (classification and synthesis methods) and glass-ceramics (devitrification and soft chemistry); their properties and applications are also discussed. Thefourth part is dedicated to metals: properties of metals and metal alloys; metal nanoparticles; and catalytic materials. Part5 is devoted to inorganic materials developed for energy; ceramics (oxides and non-oxides; nanostructured) and metal hydrides are described (properties and synthesis) through several examples and in the context of their applications (accumulators, hydrogen storage, and carbon dioxide capture).
Hourly volumes:
CM: 1:00 p.m.
Tutorial: 7 hours
Solutions, colloids, interfaces
Level of education
Bachelor's degree
ECTS
2 credits
Training structure
Faculty of Science
This course unit enables students to acquire basic knowledge and cross-disciplinary skills in the field of colloids and interfaces, which are common to the various tracks of the Master's degree in Chemistry (Materials Chemistry, Separative Chemistry, Materials and Processes, ICAP Cosmetics Engineering, Biomolecular Chemistry). It is also offered to international students enrolled in the SFRI program at the University of Montpellier, where courses are taught in English. An introductory presentation on basic notions and concepts will enable students to discover and better understand the main physicochemical properties of colloidal dispersions, associative colloids, and macromolecular solutions, as well as the parameters and phenomena governing stability in colloidal dispersions and mixed solution-colloid systems. This will be followed by interdisciplinary practical teaching based on the flipped classroom principle to help students build and deepen their knowledge through individual and collective analysis of the various applications of colloidal and interfacial phenomena and systems.
Hourly volumes:
CM: 7
TD: 13
Liquid NMR spectroscopy and X-ray diffraction
Level of education
Bachelor's degree
ECTS
2 credits
Training structure
Faculty of Science
NMR:
Liquid-phase NMR (Nuclear Magnetic Resonance) is an essential spectroscopic analysis method for chemists, enabling them to determine the structure of small organic molecules or macromolecules in solution, study dynamic phenomena, and more. The aim of this course unit is to understand the phenomena involved in this technique and to relate them to the various structural information accessible by this method. The goal is to be able to use the spectral data from this analysis to elucidate the structure and stereochemistry of organic molecules or polymer structures, or to monitor reactions.
X-ray diffraction:
X-ray diffraction is a powerful, non-destructive technique for characterizing the crystalline structure of materials. It can also provide crystallographic and structural information such as lattice parameters and atomic positions. This includes all crystallized materials such as ceramics, materials for energy and information storage and conversion, as well as organic molecules and metal complexes (interatomic distances and angles, stereochemistry (chirality, stereoisomerism, etc.), intra- and intermolecular bonds, etc.). The objective of this course unit is to provide an introduction to crystallography and diffraction, with the aim of understanding the operation and characteristics of an X-ray diffractometer, as well as interpreting diffraction patterns (structural analysis, lattice parameters).
Hourly volumes:
CM: 10
TD: 10
Chemometrics, statistical data analysis, experimental design
Level of education
Bachelor's degree
ECTS
2 credits
Training structure
Faculty of Science
This course will cover the fundamental concepts and practical tools related to chemometrics through: - statistical data analysis;
- the laws of probability;
- confidence interval estimation;
- parametric and nonparametric tests.
An introduction to design of experiments will be offered at the end of the module.
Hourly volumes:
CM: 7 a.m.
TD: 1:00 PM
Methodology for characterizing materials
Level of education
Bachelor's degree
ECTS
2 credits
Training structure
Faculty of Science
The program of this EU focuses on describing the principles and applications of the main methods for the structural characterization of solids, thin films, surfaces, and interfaces, as well as several examples of applications in materials chemistry. It includes the following techniques.
- Introduction to solid-state NMR (NMR signal, interactions in solid-state NMR, magic angle spinning, NMR sequences, cross polarization, instrumentation, etc.)
- Electron microscopy: principles and applications of scanning and transmission electron microscopy and related techniques (EDS microanalysis).
- Spectroscopic methods: Raman spectroscopy, photoelectron spectroscopy, X-ray spectroscopy (XAS, XRF, etc.), Mössbauer spectrometry.
Hourly volumes:
CM: 10 a.m.
Tutorial: 10 a.m.
Coordination chemistry and organic chemistry
Level of education
Bachelor's degree
ECTS
2 credits
Training structure
Faculty of Science
This teaching unit is dedicated to deepening the foundations of organic chemistry and coordination chemistry covered in L3 and to acquiring concepts related to molecular engineering and molecular chemistry. The teaching unit consists of lectures and tutorials. Students will prepare for certain lectures and tutorials using course materials provided, enabling them to participate fully in the lectures and tutorials, understand the concepts presented, and acquire the necessary skills. The progression program and activities will be proposed. For students who have not studied the basics of coordination chemistry and organic chemistry, documents will be made available.
Coordination chemistry: The course will cover various aspects of transition metal and lanthanide complexes, molecular materials (polynuclear complexes and coordination polymers with extended structures (MOFs, etc.)) as well as their properties and applications. Structural aspects, bond descriptions, properties, and aspects related to stability and reactivity will be addressed. Emphasis will be placed on the complexation effect and the stability of metal, lanthanide, and actinide complexes with certain ligands for applications in the biomedical field (imaging and therapy), decontamination (nuclear field), etc. The electronic (relaxivity, magnetism) and optical (absorption, luminescence) properties of these complexes will be discussed and placed in the context of applications in various fields, such as imaging, electronics, sensors, etc.
Organic Chemistry: The course builds on the knowledge acquired in the Bachelor's degree and will use a reasoned study approach to address the main reaction mechanisms in organic chemistry, providing a common foundation for all students in the Master's in Chemistry program. The main processes (substitution, addition, elimination, transposition, etc.) and their essential characteristics and applications to mechanistic sequences will be examined. This course should provide students with general tools for analyzing mechanisms (ionic, radical, concerted) in order to understand these mechanisms in all their variety.
Hourly volumes:
CM: 1:00 PM
Tutorial: 7 hours
Professional projects – project monitoring
Level of education
Bachelor's degree
ECTS
8 credits
Training structure
Faculty of Science
The professional project bridges the gap between traditional practical work and internships in laboratories or companies. It takes the form of a supervised project consisting of placing students in a professional situation through collaborative (group) work based on carrying out a project in response to a problem set by a company, local authority, association, or academic. It is part of the core curriculum of the Master's in Chemistry and is carried out under the supervision of a member of the teaching team (academic or industrial). Conducted throughout the semester, this project aims to connect and consolidate the knowledge and skills acquired during the Bachelor's and early Master's programs through this professional situation. These scenarios will be directly related to the Master's program chosen by the students. In addition to chemistry-specific skills, other interpersonal, organizational, and communication skills intrinsically linked to project management will also be acquired, equipping students for their future professional lives.
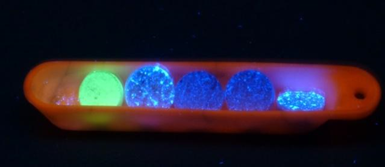
Addressing a research issue: example of a summary of new phosphorescent materials.
Hourly volumes:
CM: 5 hours
Tutorial: 5 hours
Practical work: 40 hours
Chemistry at the scale of indicators - Radiochemistry
Level of education
Bachelor's degree
ECTS
2 credits
Training structure
Faculty of Science
This teaching unit covers various aspects related to radiochemistry and chemistry at the indicator level. After describing the chemical properties of radioelements and discussing the scale factors related to the use of radioelements/radionuclides at the indicator scale, the concepts of microcomponents and macrocomponents will be addressed, as well as the kinetic and thermodynamic consequences on the development of reactions. Next, the various commonly used radiochemical methods will be introduced: extraction and purification methods, use of radioactive cows, electrodeposition, syncrystallization, or entrainment precipitation methods, isotopic labeling and dilution.
Hourly volumes:
CM: 12 p.m.
Tutorial: 8 hours
2-4 month internship (bibliography included)
Level of education
Bachelor's degree
ECTS
10 credits
Training structure
Faculty of Science
A 2- to 4-month internship must be completed in a research laboratory or a company specializing in extractive or separative chemistry, recycling chemistry, radiochemistry, materials chemistry, or process chemistry. Students will therefore have the opportunity to complete this end-of-study internship in academic research laboratories or industrial establishments. Subject to prior approval by the teaching team (internship topic related to the master's program and adequate environment/resources), students may seek a host team in an academic setting at the institutes of the Chemistry Department of the University of Montpellier, in academic laboratories outside the University of Montpellier (in France or abroad), or in the private sector (in France or abroad).
This internship, lasting between two and four months, will begin in early May and will be preceded by the submission of a bibliographic report related to the internship topic and an oral defense before a jury.
Innovative synthesis and extraction processes
Level of education
Bachelor's degree
ECTS
2 credits
Training structure
Faculty of Science
This teaching unit is shared by MI students in the Master's in Chemistry program: ICAP P1, ICAP P2, MAT P1, MAT P2, and BM (semester S2) courses. The following topics will be covered:
- The 12 Principles of Green Chemistry and units of measurement in Green Chemistry;
- Synthesis strategies in sustainable chemistry;
- Alternative or eco-friendly solvents for synthesis and extraction;
- Unconventional activation techniques and applications.
CM: 13
Tutorial: 7 hours
Radiation protection / radiation-matter interaction
Level of education
Bachelor's degree
ECTS
2 credits
Training structure
Faculty of Science
In the first part of this teaching unit, a general approach to radiation-matter interactions will be developed by addressing the different interactions and associated detection methods. A second part will develop all the concepts of radiation protection through the effects of radiation on living matter as well as the appropriate means of protection for humans and the environment.
Hourly volumes:
CM: 12 p.m.
Tutorial: 8 hours
Fundamentals of Process Engineering
Level of education
Bachelor's degree
ECTS
2 credits
Training structure
Faculty of Science
The goal of this course is to enable students with a background in chemistry to understand the fundamentals of process engineering.
The course consists of two main parts that are illustrated by the same process.
In the first part of the course, a drying process will be used to introduce the most common heat and mass transfer phenomena found in process engineering, from which the dimensionless numbers can be derived. In the second part, the thermodynamic properties of the air/water vapor mixtures will be used to derive basic dimensioning rules for the same drying process.
This course will be taught entirely in English.
Hourly volumes:
CM: 10
TD: 10
Hybrid and structured materials
Level of education
Bachelor's degree
ECTS
2 credits
Training structure
Faculty of Science
Hybrid materials are a new family of materials combining organic ligands that connect inorganic entities, and are increasingly being studied at both a fundamental and applied level.
As part of this course unit, two main categories of hybrid materials will be covered:
- Coordination Networks and Metal-Organic Frameworks
- Organosilicon/carbon materials
CM: 10 a.m.
Tutorial: 10 a.m.
Containment materials
Level of education
Bachelor's degree
ECTS
2 credits
Training structure
Faculty of Science
A general approach to confinement materials will be developed during this teaching unit, addressing the desired properties, the different classes of confinement matrices, and the associated synthesis methods. The structure-property relationships related to the confinement of radionuclides and/or toxic chemical elements will also be described. The materials covered will be glass, glass-ceramic, or ceramic.
Hourly volumes:
CM: 12 p.m.
Tutorial: 8 hours
Liquid-liquid extraction: kinetics and thermodynamics
Level of education
Bachelor's degree
ECTS
2 credits
Training structure
Faculty of Science
A general approach to liquid-liquid extraction will be developed through thermodynamics and kinetics concepts with a view to understanding the mechanisms responsible for extraction and the processes taking place at the liquid-liquid interface. The fundamental aspects of other types of extraction (liquid-solid, supercritical fluid, distillation) will also be addressed.
Hourly volumes:
CM: 12 p.m.
Tutorial: 8 hours
High-temperature chemistry
Level of education
Bachelor's degree
ECTS
2 credits
Training structure
Faculty of Science
In this teaching unit, a general approach to chemistry in non-aqueous solvents at high temperatures will be developed through concepts of chemical reactivity and the physicochemical and thermochemical properties of oxides, salts, and molten metals. Several case studies will be discussed, particularly in relation to the fuel cycle and recycling chemistry.
Hourly volumes:
CM: 12 p.m.
Tutorial: 8 hours
Communication and professional integration
Level of education
Bachelor's degree
ECTS
2 credits
Training structure
Faculty of Science
This EU will address, in small groups or on an individual basis, teaching tools and best practices related to communication and professional integration, through:
- assessment of knowledge, skills, competencies, interpersonal skills, and motivations;
- awareness of job search techniques;
- writing resumes and cover letters;
- rules for oral and written communication;
- job interview simulations.
Scenarios directly related to the sectors of activity targeted by the courses of the students concerned will be offered.
Practical work: 20 hours
Fuel cycle: from mining to waste management
Level of education
Master's degree
ECTS
2 credits
Training structure
Faculty of Science
This teaching unit covers the various aspects of the current fuel cycle and future nuclear cycles. It will cover concepts relating to the front end of the cycle (mineral resources, uranium extraction and purification, isotopic enrichment), the passage of fuels through nuclear reactors, and the back end of the cycle (reprocessing of spent fuel, recycling of recoverable materials and fuel remanufacturing, management of final nuclear waste). This will be followed by several aspects relating to future nuclear fuel cycles, in particular the use of unconventional resources, advanced separation concepts, and the development of fourth-generation reactors.
Hourly volumes:
CM: 3 p.m.
Tutorial: 5 hours
Coordination chemistry of f-elements
Level of education
Bachelor's degree
ECTS
2 credits
Training structure
Faculty of Science
A general approach to the coordination chemistry of f-elements will be developed through the concepts of atomistics, oxidation state, and coordination polyhedra in order to highlight the specific characteristics of f-elements. Direct comparisons will be made with the coordination chemistry of transition elements, and applications related to nuclear chemistry will be discussed.
Hourly volumes:
CM: 12 p.m.
Tutorial: 8 hours
Admission
Registration procedures
Applications can be submitted on the following platforms:
- French and European students: follow the "Mon Master" procedure on the website:https://www.monmaster.gouv.fr/
- International students from outside the EU: follow the "Études en France" procedure:https://pastel.diplomatie.gouv.fr/etudesenfrance/dyn/public/authentification/login.html


